Are acids denser than water? exploring density differences 3.3. the extent of sea ice — my jupyter book Ice types on water phase diagram
Why is ice less dense than water - YouTube
(color online) (a) the phase diagram of water and ice, including the
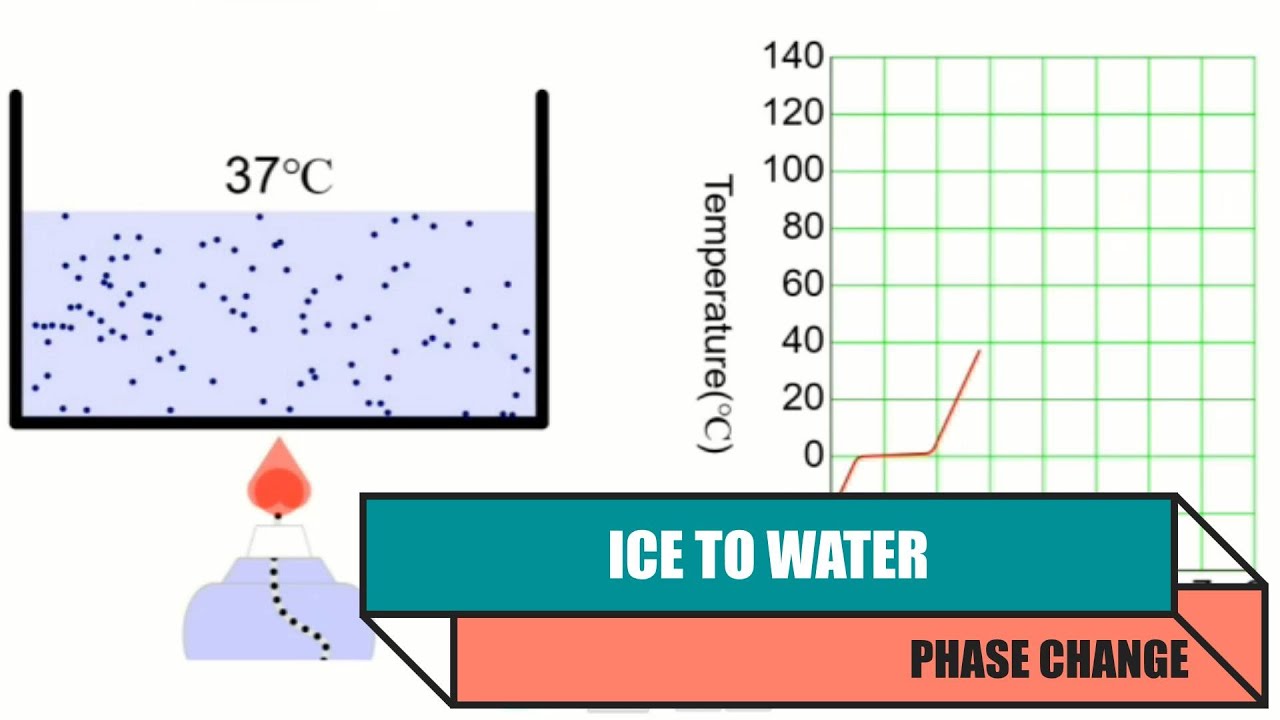
Solved the graph below shows the change of phase of ice
Phase change ratio of ice versus timePhase ice water diagram solid pressure point h2o time rule phases liquid temperature above archives tag h20 must triple gas Ice kurt water happen could vonnegut phase diagram catastropheSolved 1. consider the phase change of ice to liquid water..
Solved the phase diagram of water is unusual because itsThermodynamics of the ice-water phase transition Ice to water phase change/ physics class 9 #physics #physicsassignmentPhase pressure boiling atm h20 phases liquid pressures socratic normal 2o insanitek thermodynamics melts ranges boundary simplified temperatures linear.

Solved part a explain why ice floats in water. o ice is less
Why is ice less dense than waterLiquids generally have lower density as compared to solids. but you Secrets of the ice: thermodynamics of why salt melts iceBasic science: understanding experiments: week 2: 2.2 what if ice was.
Consider heating solid water (ice) until it becomesSolved the following diagram shows a phase change. which is Thermodynamics transition phasePhase diagram of water/ice [7].
Explain an activity to show the effect of change of temperature on the
Water phase diagram. the dark yellow arrow corresponds to immersionIce archives Phase relations between ice vii, water melt, and high-pressureFreezing water molecules.
Exploring the weight of ice and water: a scientific comparisonSolved when solid water (ice) goes through a phase The reason why ice floatsWater ice exists in several different form depending on the pressure.
Top 17 ice is denser than water 2022
Q: could kurt vonnegut’s “ice-9 catastrophe” happen?Due to what is ice less dense than water? Dense socraticPhase changes se.
Dense solved floats transcribed1. what happens to the ice over time?2. why does this happen?3 Solved consider heating solid water (ice) until it becomes.


![Phase diagram of water/ice [7] | Download Scientific Diagram](https://i2.wp.com/www.researchgate.net/publication/328345249/figure/fig1/AS:960037677699084@1605902227264/Phase-diagram-of-water-ice-7.png)